Abstract
- Diffuse alveolar hemorrhage (DAH) is an uncommon complication in patients with systemic lupus erythematosus (SLE), and mortality remains high. In recent years, cases of DAH due to SLE treated with extracorporeal membrane oxygenation (ECMO) have rarely been reported. The authors present a case of a 43-year-old woman with SLE who had rapidly aggravating dyspnea and hemoptysis. She was diagnosed as having DAH with refractory respiratory failure and was successfully managed with veno-venous ECMO. We propose ECMO as a useful salvage therapy in patients with alveolar hemorrhage secondary to SLE who are failing conventional ventilatory support.
-
Keywords: extracorporeal membrane oxygenation; lupus erythematosus, systemic; respiratory distress syndrome, adult
Diffuse alveolar hemorrhage (DAH) is a rare, life-threatening complication of systemic lupus erythematosus (SLE).[1] Depending on the study, DAH has been reported to complicate 2-5% of all cases of SLE; the reported mortality rate is between 23 and 92%.[2] The survival rate for pulmonary hemorrhage caused by SLE is very low when it leads to acute hemoptysis with respiratory failure and hemodynamic instability. Need for mechanical ventilatory support, high Acute Physiology and Chronic Health Evaluation II scores, thrombocytopenia and renal insufficiency are factors related to increased mortality in patients with DAH caused by SLE.[3] In spite of recent advances in anti-inflammatory therapy in SLE, mortality in patients with DAH has not improved under conventional treatments.[4] Recently, the use of extracorporeal membrane oxygenation (ECMO) as rescue therapy for refractory hypoxemia secondary to DAH caused by SLE has been reported in a few cases around the world.[5,6] ECMO has been used to manage patients with severe respiratory failure. It has been previously used in the context of DAH secondary to various vasculitis or auto-immune diseases. We report the first case of a patient with severe hypoxemic respiratory failure secondary to DAH from SLE who was successfully treated with ECMO in South Korea.
Case Report
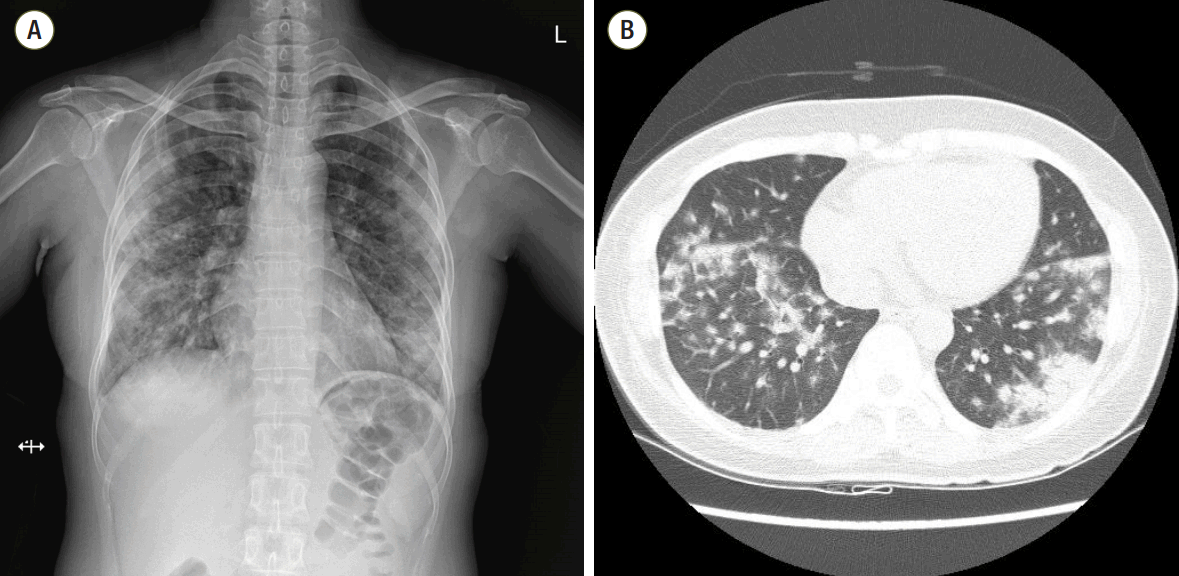
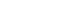
A 43-year old woman was admitted to the hospital with sudden-onset shortness of breath, cough and fever which developed 4 days ago. Approximately 1 week prior to presentation she was discharged from the hospital after undergoing a diagnostic lymph node biopsy of the right axilla. She had no other medical problems except for axillary lymphadenopathy. The lymph node biopsy led to a diagnosis of atypical lymphoid hyperplasia. Initial work-up revealed diffuse bilateral multifocal patch consolidations and a ground glass appearance in both lungs on chest X-ray imaging (Fig. 1A) and chest computed tomography (Fig. 1B). The patient received empiric IV vancomycin and pipericillin/tazobactam for bacterial pneumonia as well as intravenous (IV) methylprednisolone (62.5 mg/day) for atypical pneumonia or interstitial lung disease. Although undergoing treatment, her dyspnea, hemoptysis fever, and chest radiograph worsened. She had an increase in oxygen demand and she complained of increased hemoptysis and persistent fever. On hospital day 10, results of laboratory test showed positivity for fluorescent antinuclear antibody of 1:1280 (speckled pattern), a low white blood cell count of 2,740 cells/mm3, a low hemoglobin count of 9.8 g/dL, and a low platelet count of 70,000 cells/mm3. Other relevant labs included a positive anti-smith, positive antiribonucleoprotein, and low complement C3 and C4 levels. Based on these abnormal laboratory findings and the clinical manifestation, a diagnosis of SLE was reached. She was transferred to the rheumatology department for management of DAH caused by SLE and IV methylprednisolone (1,000 mg/day) was administered for 3 days as well as IV immunoglobulin (400 mg/kg/day) for an additional 5 days to minimize the risk of recurrent DAH. She developed worsening hypoxemic respiratory failure requiring rapid intubation and mechanical ventilator care in the intensive care unit. She underwent bronchoscopy to assess the diffuse pulmonary infiltrates seen on imaging studies. Both airways exhibited edematous and erythematous bronchial mucosa. The gross appearance with of the bronchoaveolar lavage fluid was compatible with diffuse alveolar hemorrhage.
The patient developed progressive hypoxic respiratory failure with increasing oxygen demand and required vasopressor infusion due to persistently low blood pressure. Despite various protective ventilator mode adjustments for acute respiratory distress syndrome to improve the oxygenation, a fraction of inspired oxygen (FiO2) of 1.0, and 22 cmH2O of positive end-expiratory pressure, hypoxemia worsened and oxygen saturation was consistently below 63%. Arterial blood gas analysis at that time showed pH 7.22, PaCO2 41 mmHg, PaO2 48 mmHg, and O2 saturation of 73%. Her clinical deterioration required an alternate mode of oxygenation as a rescue therapy. Therefore VV ECMO (Maquet Rotaflow Centrifugal Pumps with Quadrox-D oxygenators, Maquet, Rastatt, Germany) was initiated. A 18-French Fem-Flex II cannula (Edwards Lifesciences, Irvine, CA, USA) was inserted in the right internal jugular vein and was used as the return. A 19-French Bio-medicus cannula (Medtronic Incorporated, Minneapolis, MN, USA) was inserted in the right femoral vein and used as the drain (Fig. 2). ECMO was started after the catheters had been inserted. Initially, the oxygen fraction, sweep gas flow rate, and average flow rate of VV-ECMO were 100%, 3.6 L/min, and 3.7 L/min, respectively. Subsequently, saturation measured on pulse oximetry rose quickly to over 99% after extracorporeal circulation began. A lung protective ventilation strategy was employed.
Unfractionated heparin for systemic anticoagulation was given at the lower dosage. It was started gradually to titrate the activated clotting time (ACT) to between 140 and 160 seconds for 24 hours and then increased to maintain the ACT between 180-200 seconds. The registered activated partial thromboplastin time fluctuated between 38-55 seconds. No secondary bleeding occurred and there were no subsequent thrombotic complications. After ECMO was performed, a small amount of bleeding was observed with endobronchial suction. Procalcitonin and C-reactive protein levels were elevated. Because infection was not excluded, the patient received empiric therapy with broad spectrum antibiotics, immunosuppressive drugs such as cyclophosphamide were avoided, and plasmapheresis was performed to reduce inflammation related to highly active SLE.
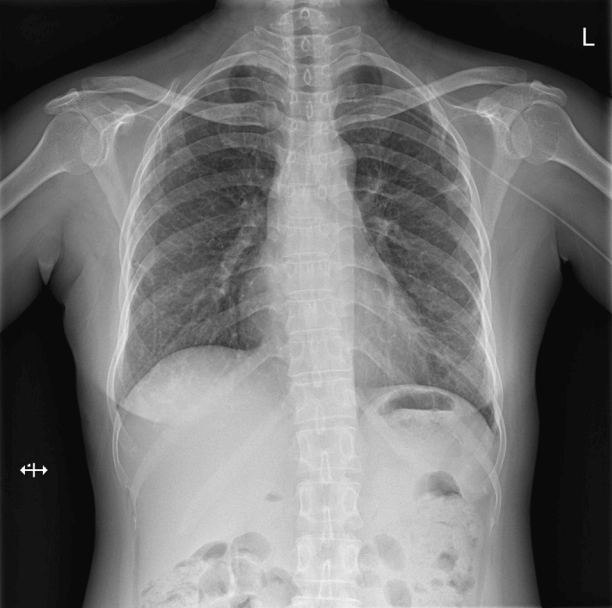
ECMO was continued for 8 days. She recovered and was successfully extubated after 13 days of mechanical ventilation. At the time of discharge, the patient could breathe normally on room air and had no neurological sequelae (Fig. 3). The patient was discharged from the hospital on oral prednisolone (50 mg/day) and she received IV cyclophosphamide administered every two weeks at an outpatient clinic.
Discussion
We report the first case of ECMO in the setting of DAH secondary to SLE disease flare-up in Korea. DAH caused by SLE is scarce and life-threatening complication and sudden-onset dyspnea is the most consistent symptom. [7] The incidence of hemoptysis varies between 25 and 100%, and the appearance of new-onset pulmonary infiltrates in both lungs and drops in hemoglobin may indicate DAH when hemoptysis is absent.[7] Death results from respiratory failure due to massive DAH in most patients despite treatment with pulsed methylprednisolone or other immunosuppressants. However, immediate institution of ECMO could increase oxygenation and it makes it possible for the patient to withstand flare-ups of SLE as a bridge therapy, as seen in our case.[5,6] In recent years, there are reports of ECMO as a useful salvage therapy for refractory respiratory failure in patients with DAH caused by SLE.[5,6]
ECMO is generally used as rescue therapy for cases where conventional ventilation and hemodynamic support measures have failed to improve the patient’s clinical condition. Formal indications for ECMO are severe hypoxemia with a PaO2/FiO2 of < 100 mmHg despite optimal ventilator settings or an alveolar arterial gradient of more than 600 mmHg in the absence of cardiogenic pulmonary edema or hypercapnia with a pH of less than 7.20.[8]
ECMO is contraindicated in cases of irreversible respiratory or cardiac disease. The case we describe involved acute respiratory failure that did not respond to conventional ventilatory support. Given the patient’s age and her potentially reversible underlying conditions (severe flare-up of SLE and systemic infection), she was a candidate for veno-venous ECMO. Complications of ECMO include thromboembolism, local infection and, less commonly, technical problems related to cannulation.[8] Complications such as hemorrhage often result from the need for systemic anticoagulation with heparin in order to prevent thrombosis. For this reason, ECMO is often relatively contraindicated in patients at high risk of bleeding.
Patients with DAH also are particularly vulnerable to increased bleeding risk. Despite the increased risk of ECMO circuit thrombosis, heparin for anticoagulation therapy should be withheld initially due to hemorrhage risk until hemostasis is achieved. Advanced ECMO circuit and oxygenation membrane technology can be used for short periods without anticoagulation.[9] Once hemostasis is achieved, systemic anticoagulation therapy with heparin should be started to reduce the risk of thrombosis in the ECMO circuit and oxygenation membrane. In this case of SLE-induced DAH,[6] systemic anticoagulation was initiated upon the installation of ECMO. There were no hemorrhagic complications related to ECMO.
In one case series of pediatric patients with DAH secondary to multiple baseline pathologies, patients were anticoagulated, although at slightly lower clotting time levels, and they did not have hemorrhagic complications. [10] Pediatric patients with severe respiratory failure due to DAH can be successfully treated with ECMO with a continuous heparin drip at the targeted ACT range of 160-180 seconds.[10] Using anticoagulation with the lower standard clotting time parameters reported in this case should be considered in adult patients with DAH.
ECMO is good choice for rescue therapy in the setting of severe respiratory failure in alveolar hemorrhage caused by SLE. When patients with refractory hypoxemia are not responsive to conventional ventilation strategies, ECMO can improve oxygenation and may improve the clinical outcomes of pulmonary hemorrhage. The use of ECMO may reduce further lung injury related to high airway pressure and oxygen toxicity while allowing more time for aggressive anti-inflammatory therapy. [9] When inflammation regulation against SLE is started immediately, these interventions should be successful. The treatment strategy for DAH caused by SLE in adults is combination therapy using high-dose glucocorticoids and cytotoxic drug such as IV cyclophosphamide[11] and plasmapheresis.[12] As in our case, simultaneous use of ECMO and plasmapheresis has a advantage for sharing vascular access and has been applied for fatal alveolar hemorrhage secondary to other connective tissue diseases.[13]
Because of the rarity of this fatal condition of DAH caused by SLE, there are no standard treatment guidelines. In addition to, an agreement for the optimal target level of systemic anticoagulation for patients with severe alveolar hemorrhage should be determined. Multi-institutional trials and data collection are needed to establish whether ECMO as a rescue therapy confers better survival outcomes in patients with DAH caused by SLE; in addition, development of safe strategies for anticoagulation in these patients is warranted.
NOTES
-
No potential conflict of interest relevant to this article was reported.
Fig. 1.(A) Chest radiography at the time of hospitalization. Initial chest radiography shows multifocal patchy consolidations in both lungs. (B) Initial chest computed tomography. Chest computed tomography scan shows bilateral multifocal patchy consolidations and ground glass appearance with mild interlobular septal thickening and air-bronchograms in both lungs.
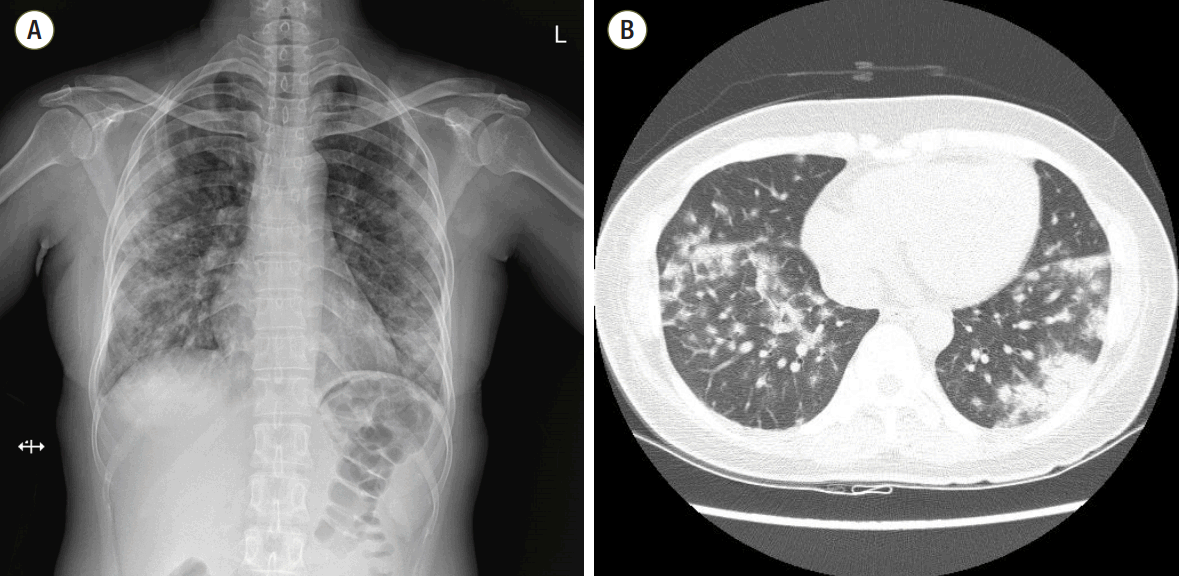
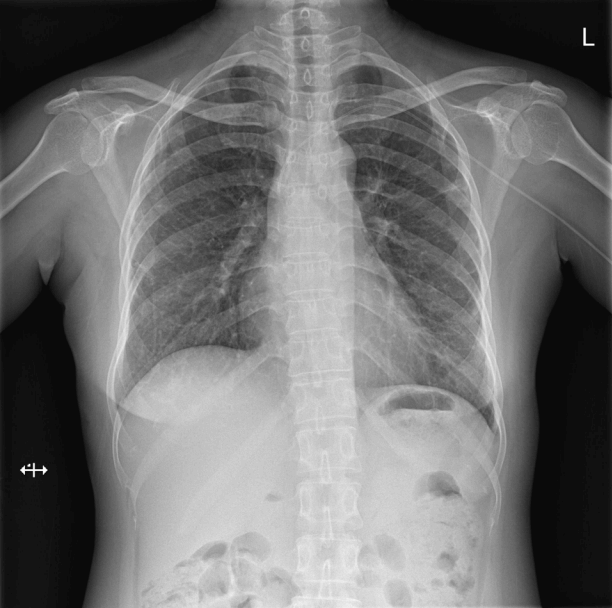
Fig. 2.Chest radiography after the initiation of extracorporeal membrane oxygenation (ECMO). Chest radiography immediately after the initiation of ECMO shows more aggravated bilateral alveolar infiltrates than seen on prior imaging studies.
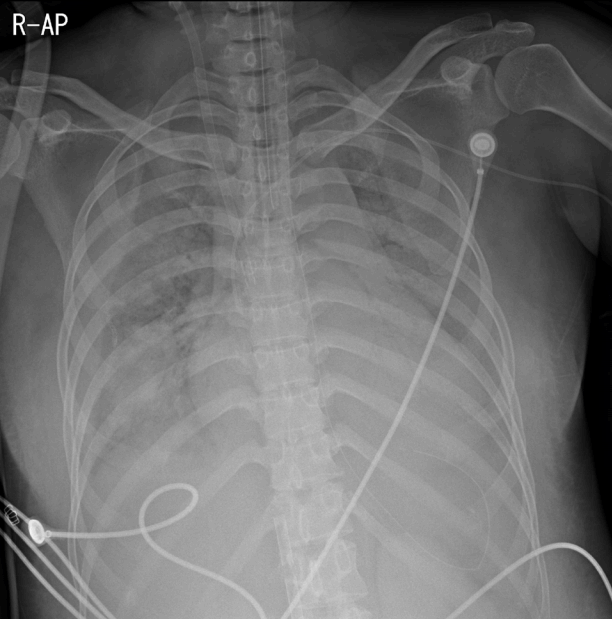
Fig. 3.Chest radiography prior to discharge. Chest radiography was performed prior to discharge and shows improvement in both lungs.
References
- 1. Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997;76:192-202.ArticlePubMed
- 2. Cañas C, Tobón GJ, Granados M, Fernández L. Diffuse alveolar hemorrhage in Colombian patients with systemic lupus erythematosus. Clin Rheumatol 2007;26:1947-9.ArticlePubMed
- 3. Martinez-Martinez MU, Sturbaum AK, Alcocer-Varela J, Merayo-Chalico J, Gómez-Martin D, Gómez-Bañuelos Jde J, et al. Factors associated with mortality and infections in patients with systemic lupus erythematosus with diffuse alveolar hemorrhage. J Rheumatol 2014;41:1656-61.ArticlePubMed
- 4. Todd DJ, Costenbader KH. Dyspnoea in a young woman with active systemic lupus erythematosus. Lupus 2009;18:777-84.ArticlePubMed
- 5. Pacheco Claudio C, Charbonney E, Durand M, Kolan C, Laskine M. Extracorporeal membrane oxygenation in diffuse alveolar hemorrhage secondary to systemic lupus erythematosus. J Clin Med Res 2014;6:145-8.PubMedPMC
- 6. Patel JJ, Lipchik RJ. Systemic lupus-induced diffuse alveolar hemorrhage treated with extracorporeal membrane oxygenation: a case report and review of the literature. J Intensive Care Med 2014;29:104-9.ArticlePubMed
- 7. Badsha H, Teh CL, Kong KO, Lian TY, Chng HH. Pulmonary hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum 2004;33:414-21.ArticlePubMed
- 8. Allen S, Holena D, McCunn M, Kohl B, Sarani B. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med 2011;26:13-26.ArticlePubMed
- 9. Ahmed SH, Aziz T, Cochran J, Highland K. Use of extracorporeal membrane oxygenation in a patient with diffuse alveolar hemorrhage. Chest 2004;126:305-9.ArticlePubMed
- 10. Kolovos NS, Schuerer DJ, Moler FW, Bratton SL, Swaniker F, Bartlett RH, et al. Extracorporal life support for pulmonary hemorrhage in children: a case series. Crit Care Med 2002;30:577-80.ArticlePubMed
- 11. Martínez-Martínez MU, Abud-Mendoza C. Diffuse alveolar hemorrhage in patients with systemic lupus erythematosus. Clinical manifestations, treatment, and prognosis. Reumatol Clin 2014;10:248-53.ArticlePubMed
- 12. Erickson RW, Franklin WA, Emlen W. Treatment of hemorrhagic lupus pneumonitis with plasmapheresis. Semin Arthritis Rheum 1994;24:114-23.ArticlePubMed
- 13. Tandon M, Reynolds HN, Borg U, Habashi NM, Cottingham C. Life-threatening acute systemic lupus erythematosus: survival after multiple extracorporeal modalities: a place for the multipotential extracorporeal service. ASAIO J 2000;46:146-9.ArticlePubMed
Citations
Citations to this article as recorded by












 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite