Abstract
-
Background
- New variants of the virus responsible for the coronavirus disease 2019 (COVID-19) pandemic continue to emerge. However, little is known about the effect of these variants on clinical outcomes. This study evaluated the risk factors for poor pulmonary lung function test (PFT).
-
Methods
- The study retrospectively analyzed 87 patients in a single hospital and followed up by performing PFTs at an outpatient clinic from January 2020 to December 2021. COVID-19 variants were categorized as either a non-delta variant (November 13, 2020–July 6, 2021) or the delta variant (July 7, 2021–January 29, 2022).
-
Results
- The median age of the patients was 62 years, and 56 patients (64.4%) were male. Mechanical ventilation (MV) was provided for 52 patients, and 36 (41.4%) had restrictive lung defects. Forced vital capacity (FVC) and diffusion capacity of the lung for carbon monoxide (DLCO) were lower in patients on MV. Male sex (odds ratio [OR], 0.228) and MV (OR, 4.663) were significant factors for decreased DLCO. The duration of MV was associated with decreased FVC and DLCO. However, the type of variant did not affect the decrease in FVC (P=0.750) and DLCO (P=0.639).
-
Conclusions
- Among critically ill COVID-19 patients, 40% had restrictive patterns with decreased DLCO. The reduction of PFT was associated with MV, type of variants.
-
Keywords: COVID-19; mechanical ventilators; pulmonary function test; risk factor; SARS-CoV-2 variants
INTRODUCTION
The number of new cases of coronavirus disease 2019 (COVID-19) continues to rise in South Korea, with critically ill cases now being reported nationwide [1]. Some COVID-19 patients require treatment in an intensive care unit (ICU) for pneumonia. Such patients develop severe clinical manifestations with acute respiratory distress syndrome (ARDS) requiring ICU admission and mechanical ventilation (MV) [2,3].
After recovery from COVID-19, patients often complain of persistent shortness of breath and are subsequently recommended for chest imaging or a pulmonary lung function test (PFT) in an outpatient clinic. PFT examination findings, after recovery from COVID-19, vary depending on the severity of the disease [4]. Patients who were critically ill during their hospital stay showed greater reductions in diffusing capacity of the lung for carbon monoxide (DLCO) [5]. A meta-analysis found that the most important indicator of PFTs after COVID-19 was DLCO [6]. However, no studies have been conducted on the risk factors for decreased lung function due to severe COVID-19 pneumonia and PFT of critically ill COVID-19 survivors in South Korea. This study analyzed PFTs of critically ill COVID-19 patients who needed a high-flow nasal cannula (HFNC) or more respiratory support and compared PFT results of patients according to the variant of COVID-19 and evaluated the risk factors associated with decreased lung function.
MATERIALS AND METHODS
Research Ethics
The study protocol was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (No. B-2012-652-111) and the study conformed to the Declaration of Helsinki (revised edition, 2013). The requirement for informed consent was waived by IRB because of the retrospective nature of the study.
Study Design and Patients
This was a retrospective, descriptive study that analyzed the clinical information of 87 survivors of COVID-19 who were critically ill due to pneumonia, treated in the ICU of a tertiary hospital in South Korea, and underwent PFT examinations after recovery from January 2020 to December 2021. According to the World Health Organization [7], laboratory confirmation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was defined as a positive result of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assays of nasal and pharyngeal swabs. The study included all patients with hypoxic respiratory failure and required at least HFNC or higher respiratory support, such as MV and extracorporeal membrane oxygenation (ECMO), for the treatment of COVID-19–induced ARDS. The study excluded the patients who survived but were lost to follow-up or who could not be subjected to a PFT due to their condition, including refusing a test. A total of 225 severe COVID-19 pneumonia patients were found in electronic medical records, and 87 patients underwent PFT (Figure 1).
The patients were categorized into two groups based on the dominant variant of SARS-CoV-2 at the time of diagnosis: a non-delta variant group (national spread period: November 13, 2020–July 6, 2021) and a delta variant group (spread period: July 7, 2021–January 29, 2022), which were announced by the Korea Centers for Disease Control and Prevention [8].
Data Collection
Patient data from the electronic medical records, prescription records, and clinical observation records were collected and analyzed retrospectively. The following data were recorded and analyzed: (1) demographics, comorbidities, and vaccination status; (2) treatment modalities and date of application of respiratory support and rescue therapy, oxygen supplement, MV settings including peak pressure, tidal volume, positive end-expiratory pressure (PEEP), Sequential Organ Failure Assessment (SOFA) index, and ratio of oxygen saturation (ROX) index; (3) laboratory findings on admission day, results of repeated PCR tests during hospitalization; (4) prescribed medications including remdesivir and dexamethasone; (5) PFT findings including spirometry and DLCO at the first follow up visit to the out-patient department. Analysis of viral negative conversion was performed based on the first identified negative results during repeated PCR tests. For the interpretation of PFTs, 0.7 of forced expiratory volume in 1 second over forced vital capacity (FEV1/FVC) was evaluated as the cut-off for obstructive lung defects, and 80% of forced vital capacity (FVC) (%) cwas evaluated as the cut-off for restrictive lung defects. DLCO was classified as severe (<40%), moderate (≥40% and <60%), mild (≥60% and <80%), and normal (≥80%) [9].
Statistical Analysis
Statistical analysis between the periods of infection was performed using a Student t-test and the association between two nominal variables was tested using the chi-square test and Fisher’s exact test. Nonparametric analysis was performed using the Mann-Whitney U-test. Univariate and multivariate logistic regression analyses were used to analyze risk factors for decreased lung function. The association of PFT with the MV application periods was analyzed by multiple linear regression analysis. Variables with a P-value <0.2 in the univariate analysis were incorporated into multivariable analysis and selected by the backward log-likelihood ratio method. A P-value of <0.05 was considered statistically significant. Statistics were analyzed using IBM SPSS statistics ver. 25 (IBM Corp.).
RESULTS
Baseline Characteristics of the Patients
The median age of the patients was 62 years (interquartile range, 28–88 years). Of the 87 patients, 56 (64.4%) were male, and 10 (11.5%) and 77 (88.5%) were ever and never smokers, respectively. Three patients (3.4%) had underlying lung disease. Age, sex, and smoking history did not differ significantly according to the variants (Table 1). MV was applied to 52 patients (59.8%) and 35 patients (40.2%) recovered from severe COVID-19–related pneumonia without MV support. MV showed no significant difference between the variants (P=0.193). There was no significant difference in the fraction of inspired oxygen (FiO2), SOFA score, and ROX index on day 1 between the MV and without MV groups (Supplementary Table 1). Rescue treatments such as inhaled nitric oxide (iNO), prone position, or ECMO were required in 29 patients during MV, of which 20 patients required only iNO or prone without ECMO, and 9 patients required iNO or prone with ECMO support. An analysis of rescue therapy also found no significant difference between the patient groups (P=0.432) (Table 1, Figure 1). There was no difference in the frequency of dexamethasone prescription (P=0.298), or duration of prescription (P=0.459), but remdesivir was more used in the delta variant spread period (non-delta vs. delta, 70.9% vs. 93.8%; P=0.011). The length of hospitalization differed according to the virus variant (36.6 days and 22.8 days, respectively, for the non-delta and delta variants; P=0.015) (Table 1).
Pulmonary Lung Function Tests
PFT was performed after 128.2 days (standard deviation [SD], 71.84 days) from initial confirmed COVID-19 with PCR test. Two patients (2.3%) showed obstructive lung defects without restrictive defects, three (3.4%) showed mixed pattern lung defects, and 36 (41.4%) showed restrictive lung defects (Supplementary Table 2). DLCO was performed in 77 patients and reported as normal in 30 (39.0%) patients and decreased in 47 (61.0%) (Supplementary Table 2). The values of the PFT were expressed as the mean±SD (FEV1/FVC, 0.82±0.07; FEV1 [% of predicted], 90.2±22.4; FVC [% of predicted], 78.7±19.2; DLCO [% of predicted], 71.1±23.1) (Table 2). When the patients were divided according to the variants, PFT showed no significant difference between the groups (Table 2). The patients who recovered with MV application had significantly worse FVC (%) (with vs. without MV, 75.3 vs. 83.6; P=0.049) and DLCO (%) (with vs. without MV, 63.9 vs. 83.6; P<0.001) when compared to patients without MV application (Table 2). The factors affecting decreased FVC (%) were analyzed after adjusting for age, sex, smoking status, and dexamethasone prescription. MV application (odds ratio [OR], 2.545; 95% confidence interval [CI], 1.037–6.249; P=0.041) was a significant risk factor in the univariate analysis but did was not significant when introduced into the multivariate analysis (Table 3). Delta variant (P=0.877) and vaccination (P=0.190) were not a significant factor in the decreased FVC (%) (Table 3). For decreased DLCO (%), male sex (OR, 0.228; 95% CI, 0.065–0.794; P=0.020) and MV application (OR, 4.663; 95% CI, 1.592–13.657; P=0.005) were significant factors in the multivariate analysis (Table 4). SOFA score (P=0.227), delta variant (P=0.532), and vaccination (P=0.561) were not significant factors in the decreased DLCO (%) (Table 4). Analyzing the ventilator application period and lung function test results, in the linear regression model, MV application periods were related to FVC (% of predicted) (B-coefficient, −0.314; P=0.013) and DLCO (% of predicted) (B-coefficient, −0.416; P=0.006) (Supplementary Table 3). The median duration of MV application were significantly different according to FVC (<80% vs. ≥80%: median duration [days], 16 vs. 6; Mann-Whitney P<0.001) and decreased DLCO (<80% vs. ≥80%: median duration [days] , 12 vs. 6; Mann-Whitney P=0.038).
With respect to the initial MV setting, higher peak pressure (non-delta vs. delta: 27.3 cm H2O vs. 23.1 cm H2O) and PEEP (non-delta vs. delta: 10.1 cm H2O vs. 8.4 cm H2O) was applied in the non-delta group but tidal volume, tidal volume/ideal body weight ratio, respiratory rate and FiO2 did not show the difference (Supplementary Table 4). Initial MV parameters did not show a significant relationship with FVC (%) (Supplementary Table 5).
DISCUSSION
This study analyzed patients who recovered from critically ill COVID-19 and determined whether there were different outcomes according to the virus variant. A recent study reported more recent SARS-CoV-2 variants were associated with a reduction in lower respiratory tract infection features [10]. Although disputed, some studies also reported the peak viral load is higher for delta [11,12]. Another study reported the delta variant showed a higher in vitro replication rate compared with alpha [13]. Structurally, the spike protein of delta more efficiently binds to the membrane angiotensin-converting enzyme 2 protein [14]. However, in our study, the decrease in lung function was related to factors such as MV rather than the virus variant. This may be because, in the severe COVID-19 pneumonia patients, lower respiratory tract infection has already progressed, so no difference according to variants, and the intensive care process and period were more affected.
Previous studies have shown that, in patients who recovered from COVID-19 and did not require oxygen supply, PFTs showed that approximately 3% of patients had restrictive lung defects, and in the patients requiring HFNC or higher devices, approximately 11% showed evidence of restrictive lung defect, calculated based on FVC (%) less than 80 [5]. There was no difference in pulmonary function as measured by PFTs before and after COVID-19 infection in non-critically ill disease [15]. In another study from Asia, 8% of patients had restrictive lung defects and 9.2% of patients had obstructive lung defects [4]. Although this study analyzed only critically ill patients who needed ICU care, we found 36 patients (41.4%) had restrictive lung defects and 47 patients (61.0%) had decreased diffusion capacity. The other study about the impact of COVID-19 on PFT showed that pneumonia severity correlates to the reduction of pulmonary function after 4 months [16]. Likewise in our study, this increase in the incidence of lung severity may be due to the difference in the severity of the disease in the patient population. Additionally, we found a higher frequency of restrictive lung defects than obstructive lung defects, which was consistent with other studies.
Our study revealed that MV was a risk factor for reduced lung function, particularly the diffusion capacity rather than FVC. The long duration of MV application associated with decreased FVC and decreased DLCO, and compared with whether or not the MV was applied, the FEV1/FVC ratio was higher than the cut-off of 0.70 in both groups, but the value was low in the group without MV application, which may be due to a lack of decrease in FVC rather than airflow limitation. It may be that the patient on MV application showed decreased lung function due to the high severity. However, as there was no difference between with or without MV application, day 1 oxygen demand or SOFA score, MV application itself may be related to lung function after recovery. A study on MV application on COVID-19 patients reported lung parenchymal involvement and MV parameters of median tidal volume/ideal body weight were risk factors of functional lung abnormality [17]. However, in our study, initial MV parameters were related to lung function. This may be because the initial settings do not represent the MV settings for the entire treatment period. MV duration may be a factor that has a greater impact on lung function, and efforts were made to apply optimal lung-protective ventilation to participants and differences in parameters might have been less-than-minimal clinically important differences. The decrease in lung function may be due to insufficient application of proper lung protective strategy during the MV even in survivors of severe COVID-19. In other words, using optimal lung protective MV would be important regardless of the etiology of respiratory failure when considering the preservation of lung function.
This study had a few limitations. First, the study was a retrospective observational study of a single center. Several multicenter prospective studies are required to evaluate risk factors for decreased lung function and analysis of clinical outcomes. Second, because there was no genetic analysis of the COVID-19 variants, the classification of patients based on the dominant COVID-19 variant at the diagnosed period may be incorrect. However, according to the published mutation information, we determined that variants such as alpha and epsilon appeared during the national spread period, and the delta variant accounted for the majority during the delta-variant spread period [8]. Third, patients who survived but were lost to follow-up or who could not be subjected to PFT due to their condition including tracheostomy or poor cooperation were not included in the study. Fourth, there was no baseline PFT before treatment; as comparison was not possible and the follow-up period was insufficient, it was impossible to analyze whether the PFT abnormality improved or persisted in the patients. Fifth, because most of the patients did not have chest radiography or chest computed tomography scans at the follow-up, there was a lack of analysis of changes in radiography and lung function according to radiography.
Notwithstanding these limitations, this is one of the few studies that reports on the factors related to poor lung function after recovery from COVID-19. We found that COVID-19 variant, vaccination status, and negative conversion of COVID-19 during hospitalization did not affect lung function after hospital discharge. Theoretically, this implies that invasive MV treatment can decrease DLCO after recovery through ventilator-induced lung injury. Well-designed studies with longer-term follow-up periods are warranted to evaluate the risk factors for decreased lung function in patients who recovered from COVID-19.
HIGHLIGHTS
▪ Approximately 40% of critically ill coronavirus disease 2019 (COVID-19) patients showed decreased lung function due to restrictive lung patterns with decreased diffusion capacity after treatment.
▪ Restrictive lung defects with decreased diffusion capacity were observed in patients requiring mechanical ventilation during hospitalization, regardless of their variant and vaccination status.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This work was supported by a National Research Foundation of Korea Grant (2021K1A4A7A02097757) funded by the Korean Government.
-
ACKNOWLEDGMENTS
The authors would like to thank the Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analysis.
-
AUTHOR CONTRIBUTIONS
Conceptualization: YJC, MJS, SYL. Data curation: THK. Formal analysis: THK, MJS. Funding acquisition: YJC. Methodology: YJC, YJL. Writing – original draft: THK. Writing–review & editing: all authors. All authors read and agreed to the published version of the manuscript.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2023.00668.
Figure 1.Application of respiratory support of the participants. COVID-19: coronavirus disease 2019; ECMO: extracorporeal membrane oxygenation.
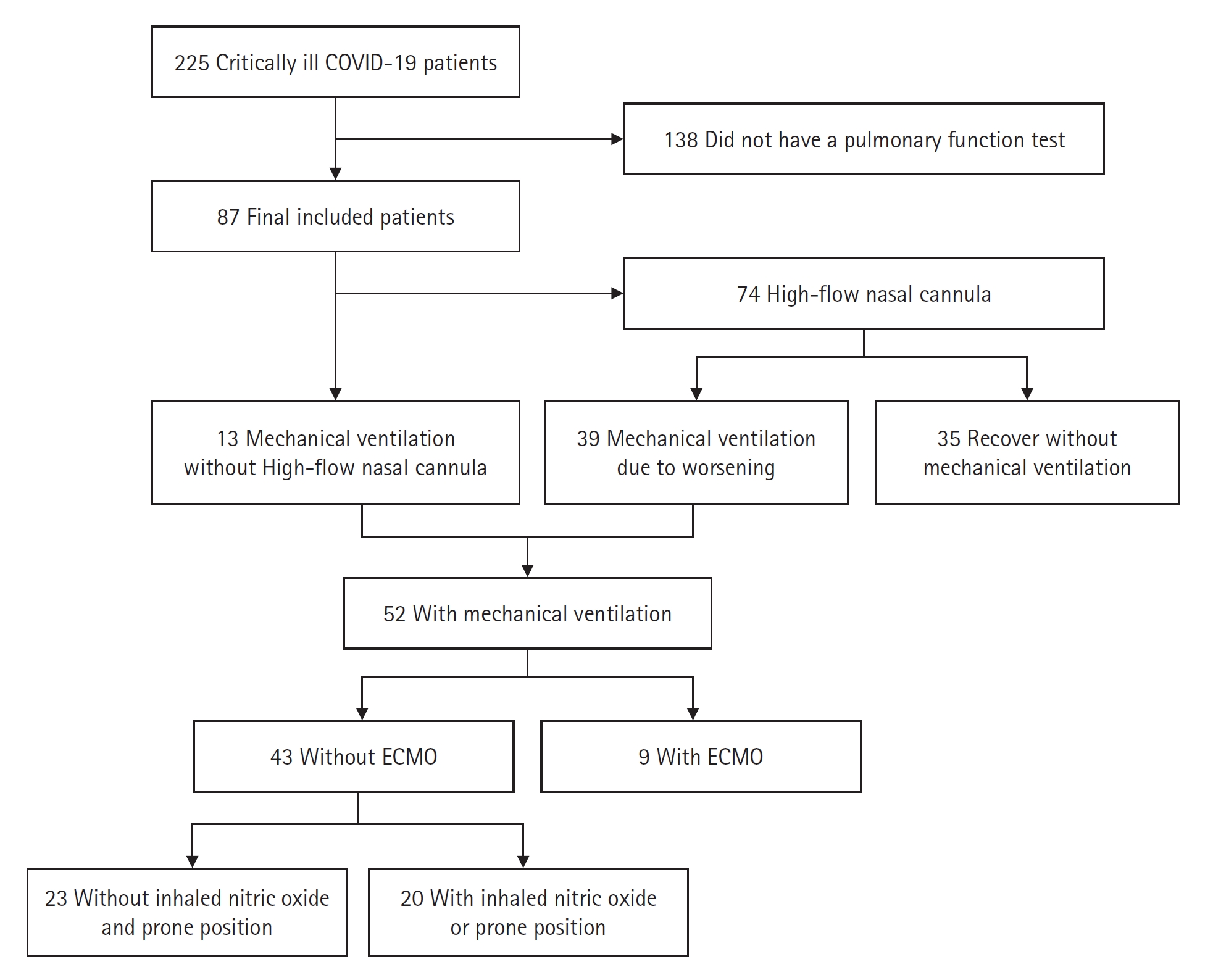
Table 1.Baseline characteristics of patients
|
Variable |
All (n=87) |
Non-delta variants (n=55) |
Delta variant (n=32) |
P-value |
|
Age (yr) |
62 (28–88) |
65 (28–88) |
57 (31–87) |
0.083 |
|
Male |
56 (64.4) |
39 (70.9) |
17 (53.1) |
0.095 |
|
BMI (kg/m2) |
26.5±4.6 |
26.8±4.6 |
26.1±4.7 |
0.535 |
|
Smoking |
|
|
|
0.062 |
|
Never smoker |
77 (88.5) |
46 (83.6) |
31 (96.9) |
|
|
Ever smoker |
10 (11.5) |
9 (16.4) |
1 (3.1) |
|
|
HTN |
47 (54.0) |
34 (61.8) |
13 (40.6) |
0.056 |
|
DM |
26 (29.9) |
19 (34.5) |
7 (21.9) |
0.213 |
|
Chronic lung disease |
3 (3.4) |
3 (5.5) |
0 |
0.550 |
|
Respiratory support |
|
|
|
0.193 |
|
Without MV |
35 (40.2) |
25 (45.5) |
10 (31.3) |
|
|
With MV |
52 (59.8) |
30 (54.5) |
22 (68.7) |
|
|
Rescue |
29 (44.8) |
20 (36.4) |
9 (28.1) |
0.432 |
|
iNO or prone only |
20 |
12 |
8 |
|
|
ECMO |
9 |
8 |
1 |
|
|
Medical treatment |
|
|
|
|
|
Remdesivir |
69 (79.3) |
39 (70.9) |
30 (93.8) |
0.011 |
|
Dexamethasone |
81 (93.1) |
50 (90.9) |
31 (96.9) |
0.298 |
|
Steroids duration (day) |
16.7±13.6 |
17.6±15.8 |
15.3±8.6 |
0.459 |
|
Laboratory test |
|
|
|
|
|
C-reactive protein (mg/dl) |
10.9±7.4 |
11.7±6.9 |
9.6±8.1 |
0.202 |
|
Procalcitonin (ng/ml) |
0.3±0.4 |
0.3±0.5 |
0.3±0.3 |
0.758 |
|
White blood cell (ⅹ109/L) |
10.1±4.8 |
9.6±4.2 |
11.0±5.6 |
0.160 |
|
Hemoglobin (mg/dl) |
13.3±3.9 |
12.9±2.2 |
13.9±5.7 |
0.253 |
|
Creatinine (mg/dl) |
0.8±0.3 |
0.8±0.3 |
0.8±0.3 |
0.930 |
|
D-dimer (mg/L) |
4.1±5.8 |
4.0±5.8 |
4.2±6.0 |
0.883 |
|
Hospitalization (day) |
31.5±25.6 |
36.6±29.5 |
22.8±13.1 |
0.015 |
Table 2.Pulmonary function tests of the patients categorized according to the variants and mechanical ventilation
|
Variable |
All (n=87) |
Non-delta variants (n=55) |
Delta variant (n=32) |
P-value |
With MV (n=35) |
Without MV(n=52) |
P-value |
|
FEV1/FVC |
0.8±0.1 |
0.8±0.1 |
0.8±0.1 |
0.239 |
0.8±0.1 |
0.8±0.1 |
<0.001 |
|
FEV1 (% of predicted) |
90.2±22.4 |
90.7±21.7 |
89.4±23.8 |
0.798 |
88.4±24.2 |
92.8±19.4 |
0.370 |
|
FVC (L) |
2.7±0.9 |
2.7±0.9 |
2.5±1.0 |
0.260 |
2.5±1.0 |
2.9±0.8 |
0.074 |
|
FVC (% of predicted) |
78.7±19.2 |
79.2±18.7 |
77.8±20.2 |
0.750 |
75.3±19.5 |
83.6±17.7 |
0.049 |
|
DLCO (ml/min/mm Hg) |
13.9±5.2 |
14.0±4.9 |
13.8±5.8 |
0.907 |
13.0±5.3 |
15.5±4.6 |
0.044 |
|
DLCO (% of predicted) |
71.1±23.1 |
72.1±23.0 |
69.5±23.7 |
0.639 |
63.9±22.3 |
83.6±18.9 |
<0.001 |
Table 3.Risk factors for decreased FVC (%)
|
Variable |
Univariate |
Multivariate |
|
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
|
Age |
0.968 |
0.933–1.004 |
0.081 |
0.975 |
0.934–1.017 |
0.238 |
|
Male |
1.818 |
0.737–4.486 |
0.194 |
1.545 |
0.574–4.158 |
0.389 |
|
Ever smoking |
0.324 |
0.117–2.029 |
0.324 |
- |
- |
- |
|
SOFA score |
0.960 |
0.804–1.147 |
0.655 |
- |
- |
- |
|
Remdesivir |
0.981 |
0.345–2.785 |
0.971 |
- |
- |
- |
|
Dexamethasone |
4.419 |
0.494–39.518 |
0.184 |
4.096 |
0.415–40.475 |
0.228 |
|
Delta variant |
0.933 |
0.388–2.244 |
0.877 |
- |
- |
- |
|
Vaccination |
0.424 |
0.125–1.511 |
0.190 |
0.621 |
0.162–2.375 |
0.486 |
|
Negative conversion of PCRa)
|
1.848 |
0.785–4.349 |
0.159 |
1.616 |
0.606–4.307 |
0.337 |
|
MV |
2.545 |
1.037–6.249 |
0.041 |
1.766 |
0.637–4.894 |
0.274 |
Table 4.Risk factors for decreased DLCO (%)
|
Variable |
Univariate |
Multivariate |
|
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
|
Age |
0.972 |
0.934–1.012 |
0.173 |
0.969 |
0.924–1.016 |
0.198 |
|
Male |
0.270 |
0.088–0.828 |
0.022 |
0.228 |
0.065–0.794 |
0.020 |
|
Ever smoking |
0.465 |
0.114–1.894 |
0.285 |
- |
- |
- |
|
SOFA score |
1.134 |
0.925–1.390 |
0.227 |
- |
- |
- |
|
Remdesivir |
1.535 |
0.517–4.555 |
0.440 |
- |
- |
- |
|
Dexamethasone |
1.048 |
0.165–6.668 |
0.961 |
- |
- |
- |
|
Delta variant |
0.532 |
0.521–3.533 |
0.532 |
- |
- |
- |
|
Vaccination |
0.700 |
0.210–2.329 |
0.561 |
- |
- |
- |
|
Negative conversion of PCRa)
|
1.543 |
0.614–3.877 |
0.356 |
- |
- |
- |
|
MV |
5.550 |
2.020–15.249 |
0.001 |
4.663 |
1.592–13.657 |
0.005 |
References
- 1. Song J, Park DW, Cha JH, Seok H, Kim JY, Park J, et al. Clinical course and risk factors of fatal adverse outcomes in COVID-19 patients in Korea: a nationwide retrospective cohort study. Sci Rep 2021;11:10066. ArticlePubMedPMCPDF
- 2. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574-81.ArticlePubMedPMC
- 3. Oliveira TF, Rocha CA, Santos AG, Silva LC, Aquino SH, Cunha EJ, et al. Extracorporeal membrane oxygenation in COVID-19 treatment: a systematic literature review. Braz J Cardiovasc Surg 2021;36:388-96.ArticlePubMedPMC
- 4. Eksombatchai D, Wongsinin T, Phongnarudech T, Thammavaranucupt K, Amornputtisathaporn N, Sungkanuparph S. Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: a prospective cohort study. PLoS One 2021;16:e0257040. ArticlePubMedPMC
- 5. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220-32.PubMedPMC
- 6. Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 2021;27:328-37.ArticlePubMedPMC
- 7. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020 [Internet]. World Health Organization; 2020 [cited 2024 Jan 20]. Available from: https://apps.who.int/iris/handle/10665/330893.
- 8. Yang S, Jang J, Park SY, Ahn SH, Kim SS, Park SB, et al. COVID-19 special report: COVID-19 outbreak report from January 20, 2020 to January 19, 2022 in the Republic of Korea [Internet]. Data Analysis Team, Central Disease Control Headquarters, Korea Disease Control and Prevention Agency (KDCA); 2022 [cited 2024 Jan 20]. Available from: https://www.kdca.go.kr/board/board.es?mid=a30501000000&bid=0031&list_no=719156&act=view#.
- 9. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68.ArticlePubMed
- 10. Hughes TD, Subramanian A, Chakraborty R, Cotton SA, Herrera MD, Huang Y, et al. The effect of SARS-CoV-2 variant on respiratory features and mortality. Sci Rep 2023;13:4503. ArticlePubMedPMCPDF
- 11. Huai Luo C, Paul Morris C, Sachithanandham J, Amadi A, Gaston DC, Li M, et al. Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant is associated with higher recovery of infectious virus compared to the alpha variant in both unvaccinated and vaccinated individuals. Clin Infect Dis 2022;75:e715-25.ArticlePubMedPMCPDF
- 12. Teyssou E, Delagrèverie H, Visseaux B, Lambert-Niclot S, Brichler S, Ferre V, et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect 2021;83:e1-3.ArticlePubMedPMC
- 13. Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IA, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021;599:114-9.PubMedPMC
- 14. Zhang J, Xiao T, Cai Y, Lavine CL, Peng H, Zhu H, et al. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Science 2021;374:1353-60.ArticlePubMedPMC
- 15. Lewis KL, Helgeson SA, Tatari MM, Mallea JM, Baig HZ, Patel NM. COVID-19 and the effects on pulmonary function following infection: a retrospective analysis. EClinicalMedicine 2021;39:101079. ArticlePubMedPMC
- 16. Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J 2021;58:2004015. ArticlePubMedPMC
- 17. Compagnone N, Palumbo D, Cremona G, Vitali G, De Lorenzo R, Calvi MR, et al. Residual lung damage following ARDS in COVID-19 ICU survivors. Acta Anaesthesiol Scand 2022;66:223-31.ArticlePubMedPMCPDF
Citations
Citations to this article as recorded by

 , Myung Jin Song2
, Myung Jin Song2 , Sung Yoon Lim2
, Sung Yoon Lim2 , Yeon Joo Lee2
, Yeon Joo Lee2 , Young-Jae Cho2
, Young-Jae Cho2





 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite